How to Determine Limiting Reactant
A specific problem A 200 g sample of ammonia reacts with 400 g of oxygen according to the equation 4NH_3 5O_2 4NO 6H_2O. Award winning educational materials like worksheets games lesson plans and activities designed to help kids succeed.

How To Find Limiting Reactant Quick Easy Examples Practice Problems Practice Questions Youtube
Limiting reactant are those compounds which are totally used up after completion of the chemical reaction and stop any further reaction.
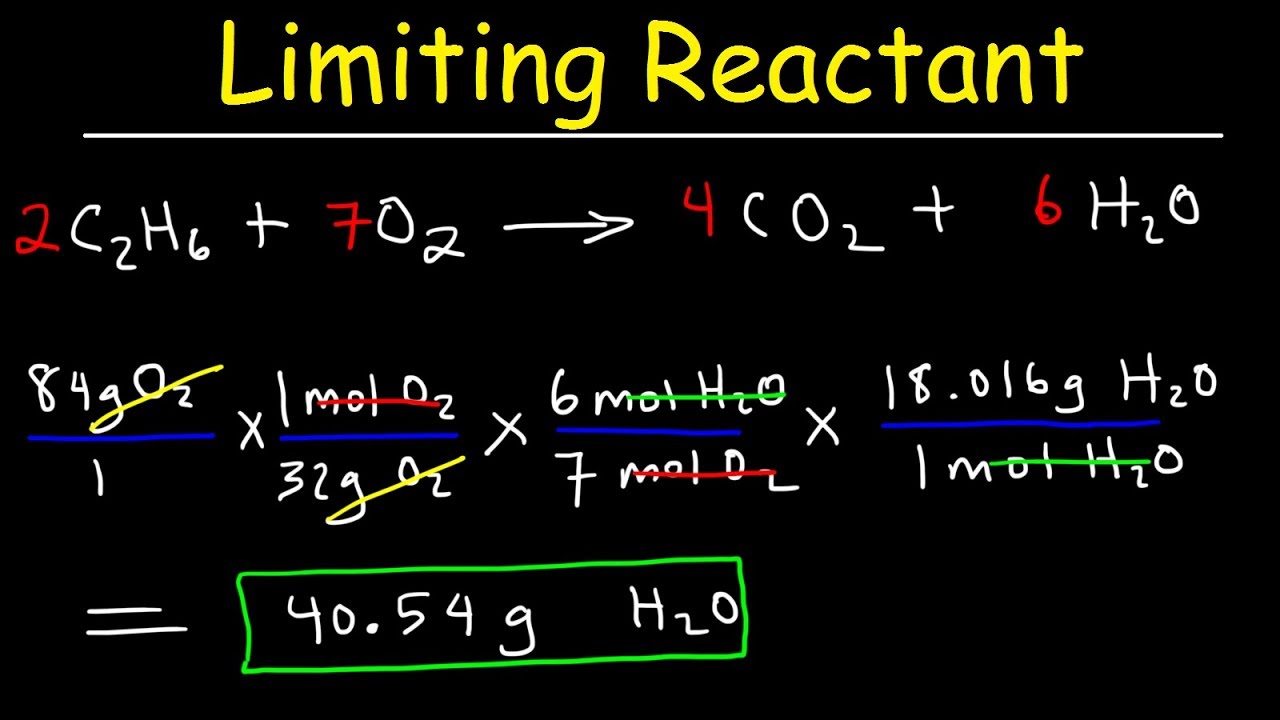
. First write a balanced complete reaction. Create a System of Equations. Use the mass molecular weight mole equation to determine the theoretical mass of the product.
As the stoichiometry of the product is 1 075 moles will form. 1 a 0b 2 c O. 150 gg of each reactant is present initiallyDetermine the limiting reactant.
The limiting reactant or reagent can be determined by two methods. The limiting reactant for the stated reaction. 23 g of sodium metal is transferred to a 3L flask filled with chlorine gas.
The amount of product formed is limited by this reagent since the reaction cannot continue without it. The determination of the limiting reactant is typically just a piece of a larger puzzle. In 1850 he began working with carbonized paper filaments in an evacuated glass bulb.
The limiting reagent will be highlighted in red. Create an equation for each element Al O where each term represents the number of atoms of the element in each reactant or product. Copy and paste this code into your website.
Suppose you have the following chemical equation and you are asked to find the limiting reactant if the amount of sodium is 25g and that of chlorine is 40g. In most of the chemical reaction two types of reactant are present. Using the mole ration.
Determine the number of moles of each reactant. We analyze several simple kinetic models to understand how the volcano curve depends on the mechanism and on the number of possible. A Al b O 2 c Al 2 O 3.
Now that we know the limiting reagent and its moles we know how many moles of the product will form. 4 C 2 H 3 Br 3 11 O 2--- 8 CO 2 6HO 2 6Br 2. By 1860 he was able to demonstrate a working device but the lack of a good vacuum and an adequate supply of electricity resulted in a short lifetime for the bulb and an inefficient source of light.
Yield is one of the primary factors that scientists must consider in organic and inorganic chemical synthesis processes. 4NH3g5O2g4NOg6H2Og Calculate the grams of product in parentheses that would be. The general problem Given the chemical equation and the masses of reactants determine the mass of excess reactant and the mass of the limiting reactant required to use up the excess.
Label each compound reactant or product in the equation with a variable to represent the unknown coefficients. If we need to determine the Molar Mass of a substance simply divide the given Mass of the Substance by the given number of Moles. Stoichiometry ˌ s t ɔɪ k i ˈ ɒ m ɪ t r i refers to the relationship between the quantities of reactants and products before during and following chemical reactions.
To determine which reactant is the limiting reactant first determine how much product would be formed by each reactant if all the reactant was consumed. This calculator will determine the limiting reagent of a reaction. Following solved examples clearly illustrate the methods elaborated in the preceding section.
Use uppercase for the first character. Create an equation for each element Al H Cl where each term represents the number of atoms of the element in each reactant or product. Limiting Reactants Calculating Excess Reactants 712 Calculating Reaction Yield and Percentage Yield from a Limiting Reactant 707 Calculating Percent Composition and Determining Empirical.
To calculate the limiting reagent enter an equation of a chemical reaction and press the Start button. To calculate the limiting reagent enter an equation of a chemical reaction and press the Start button. Label each compound reactant or product in the equation with a variable to represent the unknown coefficients.
Divide the actual number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. Start for free now. Lastly for finding the amount of remaining excess reactant subtract the mass of excess reagent consumed from the total mass given of the excess reagent.
Since the limiting reactant defines exactly how much of each reactant actually participates in a reaction stoichiometry is used to determine theoretical yield. The Balanced equation is. Above-mentioned steps have been demonstrated in the following solved examples.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of. The limiting reagent or limiting reactant or limiting agent in a chemical reaction is a reactant that is totally consumed when the chemical reaction is completed. In the following it is shown that the linear BEP relationship in a number of cases leads directly to volcano curves where the fundamental parameter is the dissociative chemisorption energy of the key 2 reactant.
The following procedures should be followed to determine the excess reactant and the quantity of excess reactant in a chemical reaction. The limiting reagent will be highlighted in red. In chemistry yield also referred to as reaction yield is a measure of the quantity of moles of a product formed in relation to the reactant consumed obtained in a chemical reaction usually expressed as a percentage.
The reactants should be converted to moles. Determine the limiting reagent if 764 grams of C 2 H 3 Br 3 reacts with 491 grams of O 2. The reactant that forms the least amount of product will be the limiting reactant.
A Al b HCl c AlCl 3 d H 2. Use uppercase for the first character. The amount of the excess reactant left after the completion of the reaction.
Enter any known value for each reactant. Determine the reactant which gives less quantity of products and that is called a limiting agent. In chemical reaction engineering yield.
We can solve the limiting reactant problem very easily by following the below steps. The amount of CO 2 produced. 0a 2 b 3 c.
So it turns out that the acetic acid is the limiting reagent. Create a System of Equations. In this limiting reactant problems what we determine is there is a reactant the limiting which limits the amount of product that can be obtained or produced.
Enter any known value for each reactant. Determine the limiting reagent and amount of excess reagent present if the mass of Na 23 and Cl 355. Joseph Swan 18281914 was a British physicist and chemist.
The limiting reactant would be used up before the other reactant while the excess reactant would be the one leftover after the reaction proceeded. If one or more other reagents are present in excess of the quantities required to react with the limiting reagent they. Expert Solution Answer.
In most limiting reactant stoichiometry problems the real goal is to determine how much product could be formed from a particular reactant mixture. This calculator will determine the limiting reagent of a reaction.

Limiting Reactant Practice Problems Youtube
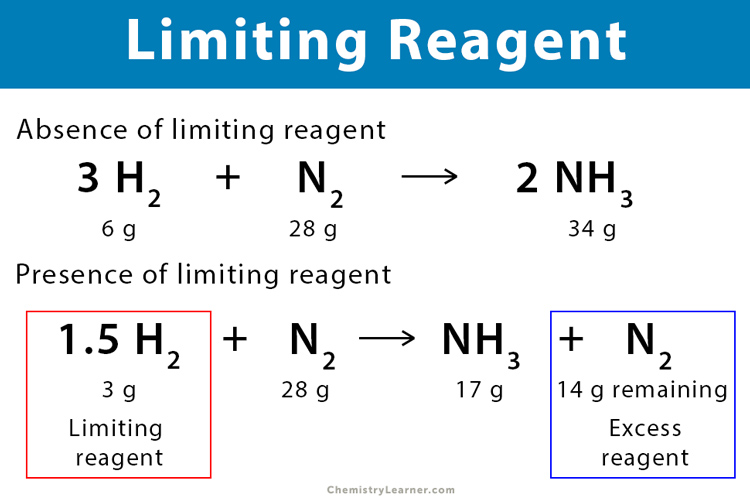
Limiting Reagent Reactant Definition Examples And Problems

How To Identify The Limiting Reactant In A Drawing Of A Mixture Chemistry Study Com

Chem 101 Dimensional Analysis Limiting Reagent Theoretical Yield Percent Yield Excess Reactant 2 Youtube
No comments for "How to Determine Limiting Reactant"
Post a Comment